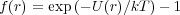
Just as the ideal gas equation of state PV = nRT assumes no interactions between the constituent atoms or molecules of a gas, so to have we assumed no energetic interactions between the monomers of our polymer. The Van der Waals equation of state results from correction terms to the ideal gas EOS based on physical excluded volume and energetic interactions which decrease (or increase) the pressure of the gas relative to the ideal state. Here, we will treat real polymer chains via consideration of excluded volume, derived by comparison with the non-interacting or ideal case. The Mayer f-function f(r) describes the probability of locating a monomer at r, relative to the non interacting system where U(r) = 0:
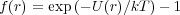 | (1) |
The excluded volume, v, is defined as
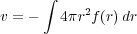 | (2) |
The excluded volume v is negative for systems with net attractive interactions, and positive for those with net repulsive interactions.