|
Graduate Student Office: 226 Mason Lab Phone: (203) 436-4059 |
Since June 2008, PhD student, Yale University, School of Engineering & Applied Science, Department of Chemical Engineering June 2007, MSc in Chemistry, Warsaw University, Chemistry Department, MSc thesis: “Synthesis, size sorting and surface modifications of magnetic ferrite nanoparticles” July 2008, MSc in Biology, Warsaw University, Biology Department, MSc thesis: “The analysis of transcription and maturation regulatory elements for snoRNA in Saccharomyces cerevisiae” June 2005, BSc in Biology, Warsaw University, Biology Department, BSc thesis: “Biosynthesis of small nuclear and small nucleolar RNAs in yeast Saccharomyces cerevisiae ” |
||||
|
||||
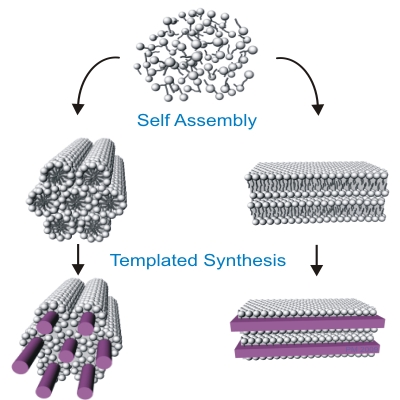
Figure 1 |
||||
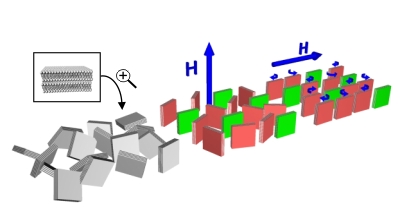
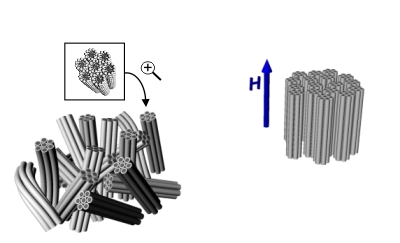
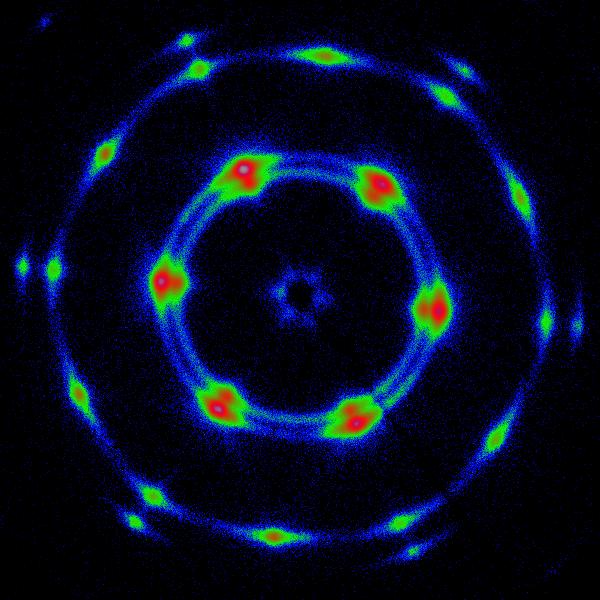
My recent studies in Prof. Chinedum Osuji's group are focused on lyotropic mesophases that may be formed by self-assembly of both ionic and non-ionic surfactants. These ordered soft materials can be directly used to sequester nanomaterials or as micro-reactors for the in-situ templated synthesis via common solvochemical routes. Figure 1 schematically visualizes the concept of the use of two different types of surfactant mesophases as matrices for synthesis of nanomaterials. The actual morphology of the sample depends on chemical properties of the system i.e.: type and concentration of used surfactants, solvent, presence of other components and can also be strongly altered by temperature. However challenging, the complexity of this systems allows for the fine tailoring of desired properties satisfying the requirements of target material and synthetic route. Two commonly encountered lyotropic mesophases, suitable for synthetic applications are hexagonal (left) and lamellar (right) structures. The production of monolithic well-aligned mesophases for use in materials sequestration and the templated synthesis of ordered, anisotropic nanomaterials, however, remains a significant challenge. This is exacerbated in thin film and discontinuous geometries where mechanical forces cannot be conveniently applied to produce shear alignment. Magnetic fields hold promise in this area, but to date their utility has been limited to ionic surfactants and to hexagonally ordered mesophases. Here, we show that judicious application of high magnetic fields can in fact drive diamagnetic alignment of non-ionic surfactants in both the lamellar and hexagonal phases (Figure 2. and Figure 3. respectively), leading to very highly ordered systems suitable for nanomaterials synthesis.
|
Figure 2:
Figure 3: |
|||
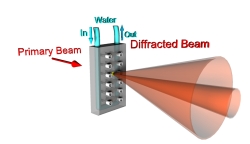
Figure 4: |
||||
Our primary research tools used to investigate the morphology and the degree of magnetic alignment of our samples are small angle X-ray diffractometry (SAXS) and polarized light optical microscopy. Our experimental design allows us to perform in situ scattering experiments on the samples exposed to static magnetic fields of controlled strength up to 6T (Figure 4.). |
||||
|
||||
|
||||
Synthesis of Magnetic Nanoparticles |
||||
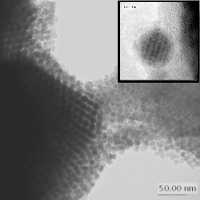
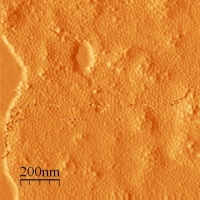
Before I was admitted to Yale Graduate School I was working in prof. Ewa Gorecka lab group in Structural Research Laboratory at University of Warsaw. I was involved in the project of synthesis of magneto-responsive materials composed of metallic cobalt nanoparticles grafted with mesogenic molecules via thiol linkage. Co nanoparticles were synthesized by thermal decomposition of carbonyl precursor in the presence of binary surfactant mixture. Figure 5. shows the TEM picture (taken by Jolanta Borysiuk) of a 3D assembly of cobalt nanoparticles evaporated from toluene on holey carbon grid. The inset reveals the crystalline structure of highly magnified particle. Hydrophobic nature of such synthesized particles allows them to form densely packed Langmuir-Blodgett type arrays when distributed over the free surface of water. These arrays can be transferred onto the solid substrate i.e. hydrophobized mica to obtain magnetic monolayers. AFM picture of such a layer is shown in Figure 6. |
Figure 5: |
Figure 6: |
||
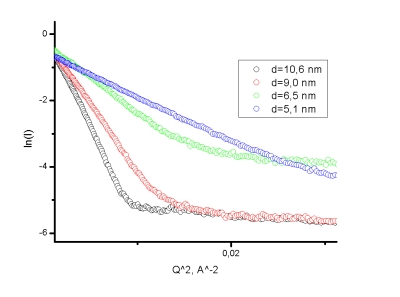
Proper adjustment of reaction conditions allows one to control the particles' size within the range from 2 to 15 nm. The mean size and polydispersity of nanometric colloidal systems can be determined with SAXS technique. The commonly used Guiner plot representing the low Q scattering profiles of samples with various cobalt grains size is shown in Figure 7.
Figure 7: |
||||
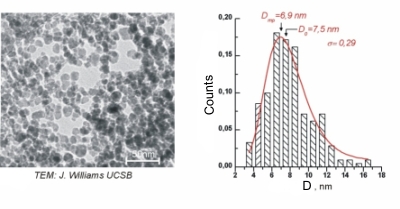
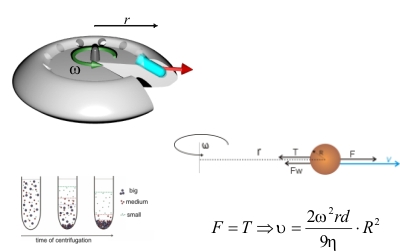
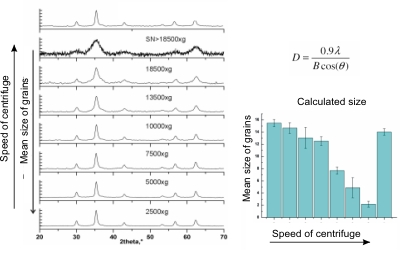
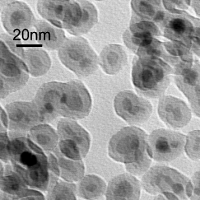
During my master's degree research I was working in prof. Pawel Krysinski group, Chemistry Department of University of Warsaw , on synthesis and characterization of nanometric ferrites. Unlike Co nanoparticles which are fairly monodisperse, ferrite nanoparticles display broad polydispersity (Figure 8.). This disadvantage is however balanced by the simplicity, low costs and big yields of aqueous precipitation technique used for their sythesis along with the good control of chemical composition allowing the tunability of magnetic properties. One of main goals of this project was to address the polydispersity problem and to find an efficient way to size separate ferritic grains. Another one was to chemically modify their surface to make them suitable for further applications i.e. for targeted drug delivery. The size sorting can be obtained relatively easily by step-by-step centrifugation process with various rotational speed (Figure 9.). The efficiency of separation was verified with powder diffractometry and SAXS studies. Diffraction peaks' broadening analysis is shown in Figure 10. My research in Poland was the continuation and extension of the studies in which I was involved as an intern in University of California at Santa Barbara in prof. David Clarke group. The overall goal of that project was to obtain a magnetic-dielectric material suitable to use in high frequency operating inductive devices. This study was motivated by extraordinary properties of magnetic nanoparticles. Below certain size particles made of ferromagnetic materials become single domained (monodomain) and magnetic moments within such a particle react to the changes in external magnetic field collectively. By analogy to paramagnetic atoms and ions this phenomenon is known as superparamagnetism. Materials made of superparamagnetic materials respond very quickly to the changes in external field and unlike their bulk composition analogues don't dissipate energy during the reorientation of magnetisation vector. This property can be observed as a lack of hysteresis effect during magnetization cycle (Figure 11). As a chemist I participated in a part of the research connected with the developement of procedure utilized to coat NiZn ferrite grains with the amorphous silica layer of controllable thickness. The proper adjustment and control over this parameter was important to tune the inductive properties of material by altering the grain separation. The technique which was used was based on modified Stober's process where organic silica precursor is hydrolized directly on the surface of the colloidal grains in the presence of ammonia as a catalyst. The representative TEM picture of the NiZn ferrite sample coated wit 10 nm thick silica layer is shown in Figure 12. The aqueous or organic suspension containing ferrite nanoparticles is called a ferrofluid. When concentrated enough it can posses quite strong magnetic properties and behaves as a liquid magnet (or rather a piece of soft magnetic material because it preserves no permanent magnetization). Ferrofluid in the movie contains about 20% wt/vol NiZn ferrite and is stabilized electrostatically with tetraethylammonium hydroxide (strong base with bulky cation having low screening properties). Look it up! |
Figure 8:
Figure 9:
Figure 10:
Figure 11:
Figure 12 |
|||
Ferrofluid movie - click to play. |
||||
|
||||
|
|
||||