Nanodrops of ionic liquids
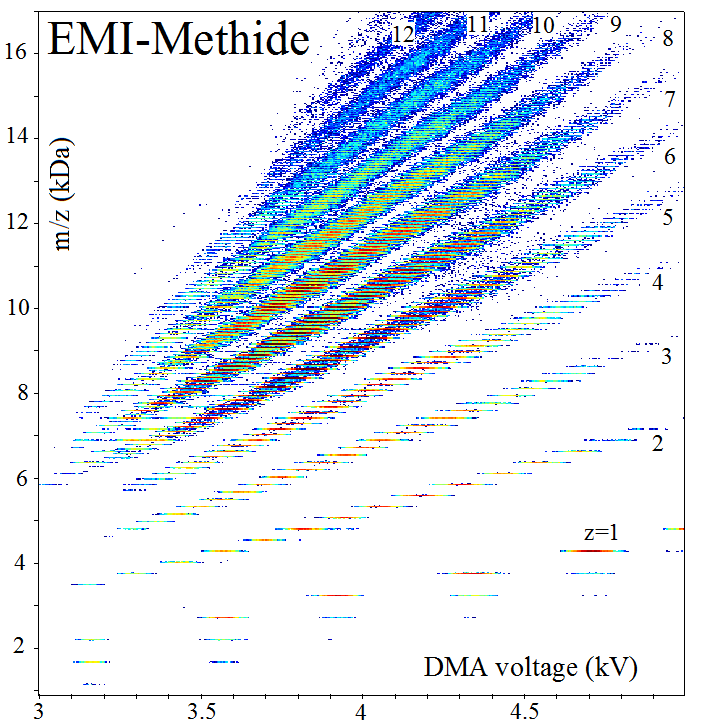 |
DMA-MS spectrum for electrosprayed nanodrops of the ionic liquid EMI-Methide. Each short horizontal segments is a peak for a pure species (n anions and n+z cations). The color scale represents particle abundance. The peaks group into broad bands with fixed charge states z (indicated), yielding mobility versus m and z, and size distributions for the various charge states. The groups of vertically displaced lines for z=1 nanodrops are due to single neutral molecule evaporation events following the DMA, greatly enhanced by the Kelvin effect (adapted from Hogan and Fernandez de la Mora, Phys. Chem. Chem. Phys., 2009, 11, 8079-8090. [reference 3 in Publications].
|
|---|
Electrosprays of ~10 mM ionic liquids in acetonitrile produce multiply charged nanodrops which have provided useful scientific information on a variety of subjects. For instance, the relation between the charge and the mass distribution of such electrosprayed nanodrops drops yields the activation (solvation) energy for ions to evaporate from charged drops [reference 1 in Publications]. Metastable transitions taking place in the radio frequency RF field within the ion guide of the MS provide information on RF heating of liquid clusters. The relation between mobility and size can be directly inferred from DMA-MS spectra of nanodrops.
