Large protein complexes
Tandem DMA-MS Analysis of the GroEL Complex
|
|---|
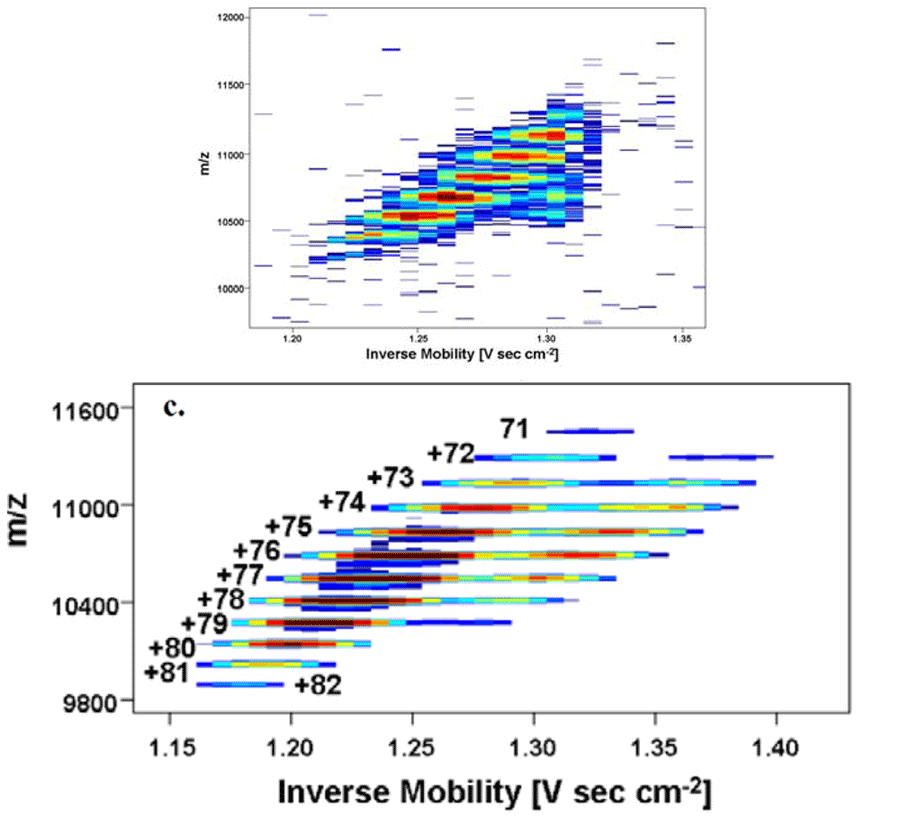
GroEL tetradecamers were purchased from Sigma-Aldrich (C7688) and purified by the Cambridge group as described by Freeke et al. (Int. J. Mass Spectrom. 2009, In Press). The GroEL complexes were dissolved in 100 mM aqueous ammonium acetate at a concentration of several μM. Sample solution was driven through a 40 mm inner diameter capillary (360 μm outer diameter, tapered down to ~60 mm at the outlet, length ~25 cm) with a backing pressure of 0.05 bar. Notice the unusually narrow mobility peaks, even though there is no declustering upstream of the DMA.